Reaction of Cyclohexene With Bromine
But liquid alkenes like cyclohexene react with bromine water solution in the. When the mixed oil is heated under pressure with copperll and chromiumlll oxides the.
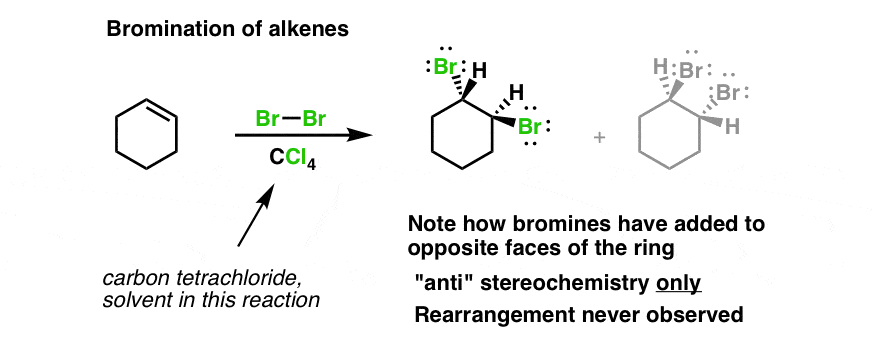
Bromination Of Alkenes Master Organic Chemistry
In recent years we have been investigating Cu-catalyzed methods for oxidation of styrenes and other alkenes for example 16 17 as well as CH bonds The former reactions are proposed to proceed via reaction of a benzylic radical intermediate with copperII generating an organocopperIII species that undergoes reductive elimination to afford a new CC or.

. In Chapter 7 we noted that alkanessaturated hydrocarbonshave relatively few important chemical properties other than that they undergo combustion and react with halogensUnsaturated hydrocarbonshydrocarbons with double or. Use only in the FUME HOOD. Halofluorination of alkenes in the presence of trihaloisocyanuric acids and HFpyridine results in the formation of vicinal halofluoroalkanes in good yields.
The main target users are workers and those responsible for occupational safety and health. Treating cyclohexene with 11-diiodoethane and a zinc-copper couple leads to tw- someric products A. The ICSC project is a common undertaking between the World.
Chelated reduction zinc borohydride. Alkenes are a class of unsaturated hydrocarbons which has a two sp2 hybridized carbon atoms meaning. Our modern society is based to a large degree on the chemicals we discuss in this chapter.
Cyclohexanol can be irritating to the respiratory system and skin. The reaction between a CC double bond and bromine Br 2 can be used as a test for the presence of alkene in an unknown sample. Cyclohexene has an.
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. To make polyamide 6 pure cyclohexanone is required. Aldol Reaction syn product.
Diels-Alder reaction will occur that forms a cyclohexene. Colour of the bromine water solution is decolourized as it reacts with ethene. Chelate Cram Addition MeMgBr.
BrF-bromine fluoride anion BrF bromine fluoride cation C v. When bromine is added to the sample if the reddish color disappears it means the sample contains an alkene. Felkin-Anh reduction LiAlH 4.
The decoloration of a solution of bromine in water is an analytical test for the presence of alkenes. The bromine reagent is in a reddish color and the product vicinal dibromide is colorless. NaBr-sodium bromide anion NaBr sodium bromide cation C v.
The reaction between cyclohexene with diiodoethane in zinc -copper couple forms two different. The reaction equation for bromine addition of ethene for example is. SiBr Silicon.
Alkynes under metal catalysts for example cobalt can also go. Keep bromine away from your skin. Aldol Reaction anti product.
Intramolecular carbonyl ene reaction. Achiral compounds with more than one stereogenic centre Meso. Intramolecular carbonyl ene reaction.
Do not breathe the vapours. Electrophilic addition to alkenes Symmetrical and Unsymmetrical. In the given reaction the dehydration occurs by the removal of hydroxy group and H from adjacent Q.
A demonstration of bromine substitution and addition reactions is helpful at this point. Cyclohexene epoxide opening axial. Aldol Reaction syn product.
Potassium permanganate is a strong oxidizing agent. The reaction is regioselective leading to Markovnikov-oriented products and the halofluorinated adducts follow anti-addition in the case of cyclohexene and 1-methylcyclohexene. Most are made from petroleum.
A more recent route to cyclohexanol is the Asahi process from benzene via its hydrogenation to cyclohexene and subsequent hydration to alcohol. Bromine causes severe burns. What are its features.
BrO-Bromine monoxide anion BrO Bromine monoxide cation C v. Reaction mass of 2-chloroethyl chloropropyl 2-chloroethylphosphonate mixture reaction mass of isomers and 2-chloroethyl chloropropyl 2-chloropropylphosphonate reaction mass of isomers 401-740-0 015-144-00-X reaction mass of pentyl methylphosphinate and 2-methylbutyl methylphosphinate 402-090-0 87025-52-3 015-145-00-5. This is more energy efficient than the other processes.
In electrophilic halogenation the addition of elemental bromine or chlorine to alkenes yields vicinal dibromo- and dichloroalkanes 12-dihalides or ethylene dihalides respectively. A simple S N 2 reaction. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
The chemical reactivity of benzene contrasts with that of the alkenes in that substitution reactions occur in preference to addition reactions as illustrated in the following diagram some comparable reactions of cyclohexene are shown in the green box. Stereoselective Aldol Reaction Cis gives Syn. Cyclohexene epoxide opening axial.
Such reaction is highly selective in stereochemistry. The reaction takes place at room temperature if the reactants are in the gaseous state ethene. In the DielsAlder reaction a cyclohexene derivative.
Do not breathe vapours and prevent contact with skin. Electrophilic Addition Addition of bromine to an alkene.

Bromination Of Cyclohexene Under Conditions Given Below Yields Ltimg Src Https D10lpgp6xz60nq Youtube
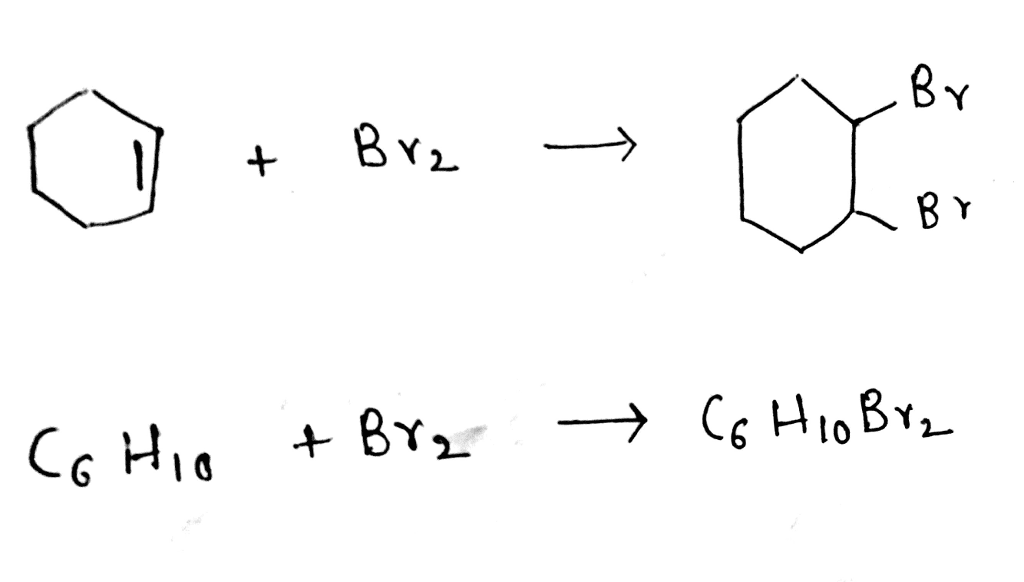
Oneclass Write A Balanced Equation For The Reaction Of Cyclohexene With Bromine
Reaction Of Bromine With Cyclohexane Cyclohexene And Benzene

Organic Chemistry Product Of Reaction Between Cyclohexene And Bromine In Methanol At 273 K Chemistry Stack Exchange
0 Response to "Reaction of Cyclohexene With Bromine"
Post a Comment